Without Fe chelates, growing high-yielding crops in hydroponics or other water culture systems would be quite difficult. The molecule DTPA is the standard chelate for iron-fertilisers in modern hydroponic systems. However, unchelated other metal cations - dissolved in a nutrient solution - can compete with Fe for the DTPA molecule.
This can result in a loss of plant-available Fe. In a scientifically set up greenhouse trial with tomato on rockwool slabs, the application of Fe- DTPA (Ultrasol® micro Rexene® FeD12) at 7 or 10 umol Fe/L resulted in a higher root zone concentration of Fe when applied with Zn, Cu and Mn chelated with EDTA, compared to co-application of their sulphate salts with Ultrasol® micro Rexene® FeD12.
The loss of chelated iron due to co-application with sulphate salts of Zn, Cu or Mn is now confirmed in a published scientific paper for crops grown on inert media like rockwool.
Reference: Bin, L.M., Moerkens, R., Noordam, A., van Aert, R. and Bugter, M.H.J. (2020). The effect of chelating Zn, Cu and Mn on plant Fe nutritional status of hydroponically grown tomato plants. Acta Hortic. 1273, 199-206. https://doi.org/10.17660/ActaHortic.2020.1273.27
Crops need iron (Fe) to produce chlorophyll, the green pigment which is essential for photosynthesis.
This is the reason why iron deficiency in plants can become visible as chlorosis – yellowing of leaves. Even less visible reductions in chlorophyll content in leaves result in lower carbon fixation and ultimately in lower marketable crop yields.
In substrate-grown crops, it is challenging to ensure a sufficient supply of Fe in a form that is available for uptake by the crop. Under practical pH levels, Fe added in the nutrient solution will precipitate. This occurs readily when the pH reaches levels over pH > 7 in soils and can already happen at pH levels > 4 in nutrient solutions or hydroponics.
The reason for this is that under neutral to basic pH conditions, Fe exists in water in largely insoluble hydroxide forms. In complete nutrient solutions the solubility of iron is even more compromised by potential precipitation with phosphates or carbonates. Therefore, the application of Fe as simple salt (for example iron sulfate) in these systems quickly leads to precipitation, rendering iron unavailable for uptake by the crop.
Since the first introduction of the use of EDTA-chelate as a stable and effective method to provide Fe to crops growing in water culture, this practice very soon became widely accepted among growers, and has been replaced since then by an improved chelate for iron, DTPA. The success of Fe-DTPA is related to its stability under a broader pH range compared to Fe-EDTA (approximately pH 1,5 – 6,5 for Fe-EDTA versus pH 1,5 – 7,5 for Fe-DTPA), providing a more secure and stable Fe supply to hydroponically grown crops.
This experiment shows that application of unchelated Mn, Zn and Cu with Fe-DTPA result in lower plant performance and lower yield. This reiterates the importance of providing correct micronutrient nutrition to crops growing on inert substrate, like rockwool or sandy soils with low CEC capacity. Not providing sufficient levels of micronutrients, or not providing specific micronutrients in the correctly chelated form can be a cause of suboptimal yields.

Figure 1. Development of SPAD-index over time. Letters denote statistically significant differences (Tukey’s range test α≤0,05). a. 7 μmol Fe-DTPA/L nutrient solution, b. 10 μmol Fe-DTPA/L nutrient solution. Sulphate: Cu, Mn and Zn added as sulphate salts, Chelate: Cu, Mn and Zn added in EDTA chelated form.
However, iron can be replaced from the DTPA chelate by other metal cations that are essential for plant growth, like copper (Cu), manganese (Mn) or zinc (Zn), when they are added in the same nutrient solution in their sulphate forms (CuSO4, MnSO4, ZnSO4). With the cations taking the place of Fe, Fe precipitates and becomes unavailable for the plant. It is recommended to apply these cations fully chelated with EDTA to prevent the loss of production associated with the lower availability of micronutrients.
In this trial, the loss of Fe and associated loss of production was compared between a nutrient solution containing all of these micronutrients in their chelated forms, and a nutrient solution where only Fe was applied as DTPA chelate. Additionally, two levels of Fe in the nutrient solution were included.
The trial was set up in a modern greenhouse at the Research Centre Hoogstraten (Belgium) on a commercially important truss tomato cultivar – Merlice - during its entire production cycle (January – November).
Plants were grafted on the rootstock Maxifort, planted in rockwool substrate slabs and a stem density of 3,3 stems/m2 was maintained.

Figure 2. Measuring SPAD-index of tomato leaves during the trial.
Trial details
The treatments were implemented in a 2-factorial design with two nutrient solution compositions (“Chelate”: all micronutrients chelated vs. “Sulphate”: only Fe chelated), and two variations of Fe concentration (7 μmol Fe/L (7Fe) vs 10 μmol/L Fe (10Fe)). All other nutrients were supplied on the advice of a crop advisor (Table 1). The pH in the nutrient solution measured over time varied between 4,8 and 5,1 in the drip, and between 5,8 and 6,2 in the drain. The treatments were applied using two separate water systems for the factor Chelate vs Sulphate, and separated gutters for the factors 7Fe vs 10Fe. The results of treatments were analysed on a total of 64 stems, divided over four production plots.
Leaf chlorophyll production was monitored on the youngest fully expanded leaf, with weekly SPAD-index measurements following a standardised protocol. SPAD-measurements are often employed for a rapid, non-destructive estimate of leaf chlorophyll concentrations based on absorbance of light of specific wavelengths by the surface of the leaf (Figure 2). In general differences in this parameter correlate well with differences in chlorophyll concentration in the leaf induced by variations in Fe supply to the plant. Additionally the concentration of Fe in the irrigation water and the drain water was analysed two times per month. Also the effect on total fruit yield, fruit weight and blossom end rot (BER) and calcium concentration in the fruit was assessed.

Figure 3. Average total fresh tomato yield and fruit weight as determined for the entire trial period. Letters denote statistically significant differences (Tukey’s range test α≤0,05)
Results highlights
Throughout the crop cycle, no Fe deficiency-related chlorosis was observed in the plants. However, the SPAD-index was higher for the “Chelate” than for the “Sulphate” treatments in each evaluation after transplant (Figure 1 a. (7Fe) and b. (10Fe)). Correspondingly, the Fe concentration in the irrigation water and the drain water was highest for the “Chelate” compared to the “Sulphate” treatments (Table 2). This illustrates that although plants may not show visual deficiency symptoms, a nutrient may still be deficient, limiting optimal growth.
The fruit yield was 4-6% higher for the “Chelate” vs the “Sulphate” treatments, with the greater difference at the 7Fe rate. This difference was mainly explained by a higher average fruit weight in “Chelate” treatments (Figure 3). The pH of the nutrient solution in this experiment was carefully controlled, with a maximal value of 6,2 measured in the drain water. In cropping systems where the water quality, fertigation units or plant physiological reactions at the root zone may lead to a more steep increase of pH in the root zone, the loss of Fe and corresponding yield loss, may be even more notable.
Incidence of BER was less than 1% in the entire trial, but as an interesting finding, the “Chelate” treatments resulted in a statistically lower BER incidence compared to the sulphate treatment at the suboptimal dose of 7Fe, corresponding to a slightly lower Ca concentration in the dry matter of fruits (Figure 4). An lowered photosynthetic activity of the plants in the 7Fe/Sulphate treatment, and correspondingly lower transpiration, may have resulted in a lower Ca translocation to the fruits, explaining this observation.
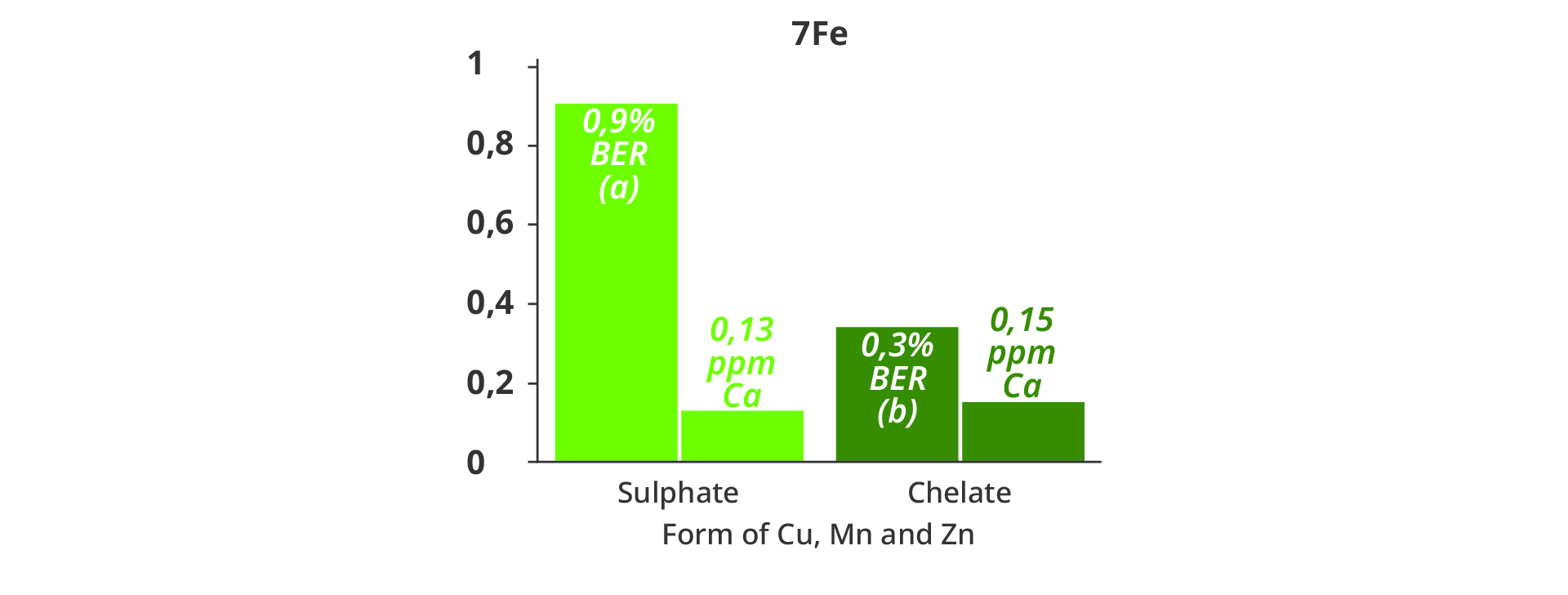
Figure 4. Average blossom end rot incidence (% BER) throughout the harvst period (Letters denote statistically significant differences (Tukey’s range test α≤0,05), and analyses of calcium concentration in the fruit dry matter (ppm Ca) at the dose rate of 7 μmol Fe-DTPA with Cu, Mn and Zn added either as sulphate salts or in EDTA chelated form.
Table 1. Applied levels of both macro- and micronutrients over time. Nutrient levels are given in mmol/L except for Fe, Mn, Zn, B, Cu and Mo which are given in μmol/L. After 7 June the levels were unchanged till the end of the trial period. *Adjusted due to Mg deficiency from the beginning of July. Agregar al título de la tabla lo siguiente: The numbers in this table are rounded off, please read the original publication for the specification in 2 decimal precision.
Table 2. Average Fe concentration (µmol/L) in the drip solution and rockwool slabs (drain water).

